As noted above, a distinctive property of sea water is its salinity (indicated by the symbol S). True salinity refers to the ratio of the mass of dissolved solids in seawater to its mass. This definition of salinity was adopted at the beginning of the last century by the International Council for the Exploration of the Seas.
Due to the complexity of the composition of seawater, it is not possible to determine by direct chemical analysis the total amount of salt dissolved in a seawater sample. The dry residue obtained after evaporation absorbs moisture very well and long heating is required to obtain it, leading to the decomposition of carbonates and magnesium salts. A group of scientists, which included M. Knudsen, K. Sorensen and S. Forch, developed a method of dehydration in a chlorine environment, which gave highly accurate results. But this method is too complicated, and in practice the salinity of sea water is never measured by it.
At present, salinity is determined either by the content of one of the components of the salt composition (the Mohr-Knudsen method), or by the electrical conductivity of sea water, or by the refractive index. As a result, different scales of salinity have arisen based on different principles. The unit of measurement of salinity depends on the method of its determination - g kg "1 or% o (ppm) in the case of the Knudsen method and in units of practical salinity when determining S by electrical conductivity.
Mohr-Knudsen method based on the constancy of the salt composition of sea water. Due to possible deviations from Dietmar's law, the determination of the total mass of dissolved salts from the content of one of the components is not entirely accurate, but not so significant compared to the accuracy of the salinity determination itself. In this method, salinity (96o) is determined by chlorine content(C1) sea water, which is the sum of ions of chlorine, bromine and iodine and obtained by the standard argentometric method of titration for chlorine. The relationship between salinity and chlorine for ocean waters is expressed by the empirical formula:
This formula was obtained in 1901 by M. Knudsen. It is valid for the salinity range of sea waters from 2.69 to 40.18% o. Note that for closed seas (Caspian, Aral), as well as inland seas (Baltic, Black, Azov), the relationship between salinity and chlorine differs from (3.34) .
The presence in (3.34) of a free term leads to the fact that salinity turns out to be a non-additive quantity. At the same time, it is known that salinity is a conservative value, subject to a linear mixing law. To eliminate this contradiction in 1962, a group of experts at UNESCO proposed the following formula (Cox scale):
? %o = 1.80655-0 %about. (3.35)
To determine salinity by electrical conductivity (see section 8.1), a Practical salinity scale, 1978(ShPS-78) . This scale is based on the empirical dependence on the electrical conductivity not of natural waters, but of solutions of standard sea water. The primary standard in this method is an aqueous solution of potassium chloride (KCl) at a temperature of 15°C and a pressure of one standard atmosphere (101325 Pa).
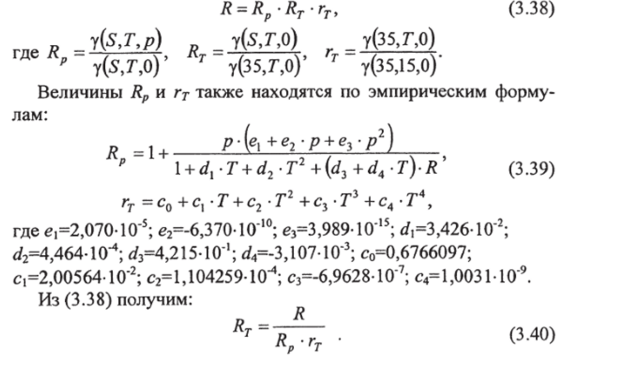
Practical salinity is calculated using the following empirical formula:
In expression (3.36) Rj stands for relative electrical conductivity, equal to the ratio of the electrical conductivity of a water sample to the electrical conductivity of water with a salinity of 35 at atmospheric pressure. Both samples should be at 15°C.
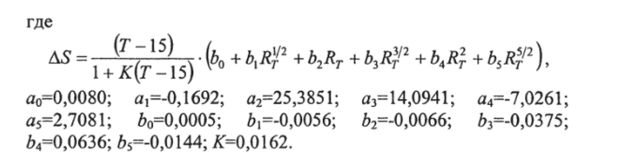
Relative electrical conductivity R T according to measurements in situ is calculated as follows. Let u (S,T,p)- conditional electrical conductivity of sea water in situ(see section 8.1), y(35.15.0) - conditional electrical conductivity of sea water at 7 \u003d 15 ° C, practical salinity 35 and atmospheric pressure (4.2914 S m "1). The value
called conductivity coefficient.
This coefficient can be decomposed into three parts:
Formula (3.36) is valid in the temperature ranges T from -2° to 35° С according to MPTS-68, practical salinity S from 2 to 42 and pressure from 0 to 1000 bar. The dependence of the practical salinity of sea water on electrical conductivity and temperature is shown in fig. 3.1.
Salinity in the World Ocean is mainly between 33 and 37%o. The exceptions are estuarine areas, desalination basins (such as the Baltic and Black Seas), where salinity decreases significantly, and salinization basins (Mediterranean and Red Seas), where salinity exceeds 38% o. The average salinity of the waters of the World Ocean is 34.72% o.
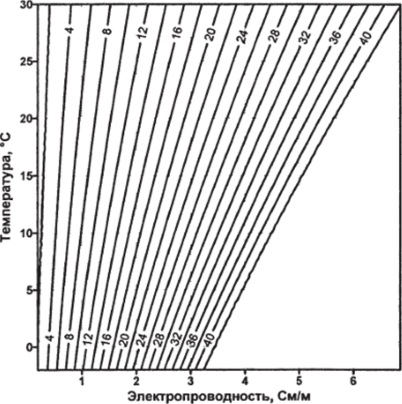
Rice. 3.1.
With depth, the salinity of water in different areas of the World Ocean varies in different ways. In the Black Sea, for example, it is increasing. In the Atlantic Ocean, it first grows (waters of increased salinity), then decreases and rises again. Table 3.4 shows the average values of water salinity in the World Ocean. If it were possible to extract all the salt dissolved in it from the ocean, then it would cover the entire globe with a layer of salt more than 40 m thick and weighing 95 tons per 1 m 2!
- Note that when writing practical salinity, the sign %o is omitted. Some domestic researchers use the abbreviation eps (practical salinity units) to designate practical salinity; in the English-language literature, this abbreviation looks like this: psu or PSS-78.
