The temperature regime determines, first of all, the rate of the freezing process.
Temperature in the range of positive and negative values affects the rate of reactions, the solubility of compounds, the rate of dissolution, coagulation, as well as the concentration of undissociated ion pairs. There are several types of temperature in solutions: structural, freezing point. Crystallization start temperature (freezing point) - the temperature at which, as a result of cooling the solution, the formation of crystals begins. Lowering the freezing point ΔТз is the difference between the freezing point of a pure solvent and a solution. The freezing point of brine is always below the freezing point of pure water and depends on the concentration of dissolved salts. This dependence for brines can be expressed by the equation:
where To- coefficient of proportionality; With is the concentration of a solute in a solution.
In less dilute solutions, the crystallization onset temperature is determined from the state diagram of the corresponding system. Since the freezing temperature of sea waters and highly mineralized natural brines will be different, we assume that this temperature should be calculated using different formulas.
We have made an approximation of the experimental data on the freezing points of solutions of table salt, sea water and natural brines used in the work. The dependences of freezing temperature changes in graphical and analytical forms are presented in Figures 41-43.
Rice. 41. The dependence of the freezing point on the salinity of a salt solution
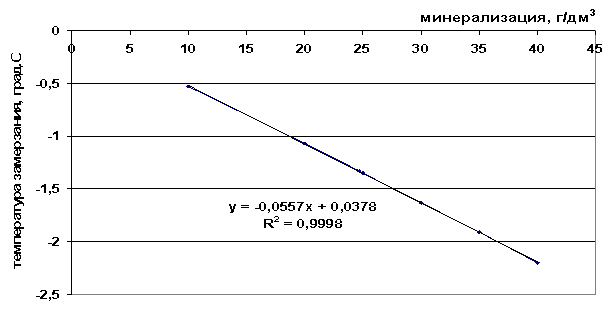
Rice. 42. Dependence of the freezing point of sea water on salinity
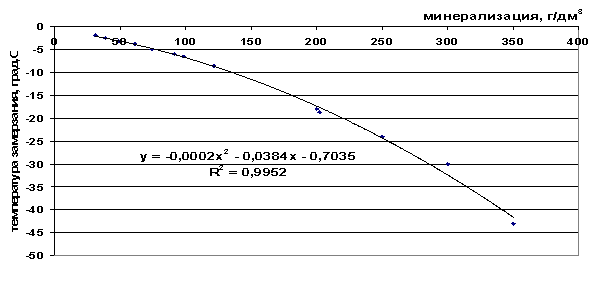
Rice. 43. Dependence of the freezing point of brine on salinity
From the presented freezing point values (Table 9) it can be seen that the freezing point decreases as the total salinity of the solution increases and as the number of components included in the frozen system increases - ΔТз(NaCl)< ΔТз(морск.вода) < ΔТз(рассол).
Table 9. Analysis of the constructed graphical dependencies
|
Ctot, g/dm 3 |
Freezing point, °С |
||
|
NaCl solution |
sea water |
||
|
t=8∙10 -5 M 2 -0.0945M+1.0595, |
0.0557M+0.0378, |
t=-2∙10 -4 M 2 -0.0384M-0.7035, |
|
* R 2 - reliability of approximation
It is known that the freezing of individual salts from desalinated water occurs at different temperatures, for example, at a temperature of -2°C, calcium carbonate precipitates. At - 3.5 ° C sodium sulfate. When the temperature drops to -20°C, table salt precipitates, to -25.5-26°C magnesium chlorides, and at very low temperatures - 40-55°C, potassium and calcium chlorides precipitate. For negative temperatures, the process of formation of crystalline hydrates, which are unstable at temperatures below 0°C, is specific. For example, hydrohalite NaCl * 2H 2 O is formed at -0.15 ° C, MgCl 2 * 12H 2 O is stable at -15 ° C, and MgCl 2 * 8H 2 O is below 0 ° C, Na 2 CO 3 * 7H 2 O is formed only at -10°C. KCl crystallizes at 0°C in the form of KCl, at -6.6°C already two phases coexist - KCl and KCl*H 2 O, at -10.6°C only KCl*H 2 O precipitates. At negative temperatures, individual crystalline hydrates with the maximum possible number of molecules of crystallization water, according to the coordination numbers at a given value, and their mixtures (but not mixed crystals). An anomalous decrease in the freezing point of concentrated solutions should be noted.
We bring to your attention the journals published by the publishing house "Academy of Natural History"
