1. Chemical properties of coal
2. Classification of hard coal
3. Formation of hard coal
4. Reserves of coal
Coal is sedimentary rock, which is a deep decomposition of plant remains (tree ferns, horsetails and club mosses, as well as the first gymnosperms).
Chemical properties of hard coal
By chemical composition coal is a mixture of high-molecular aromatic compounds with a high mass fraction of carbon, as well as water and volatile substances with small amounts of mineral impurities. These impurities form ash when coal is burned. Fossil coals differ from each other in the ratio of their components, which determines their heat of combustion. A number of organic compounds that make up coal have carcinogenic properties.
Most deposits of coal were formed in the Paleozoic, mainly in the Carboniferous period, about 300-350 million years ago. By chemical composition coal is a mixture of high-molecular polycyclic aromatic compounds with a high mass fraction of carbon, as well as water and volatile substances with small amounts of mineral impurities, which form ash when coal is burned. Fossil coals differ from each other in the ratio of their components, which determines their heat of combustion. A number of organic compounds that make up coal have carcinogenic properties. The carbon content in hard coal, depending on its grade, ranges from 75% to 95%.

Coal, a solid combustible mineral of plant origin; a type of fossil coal with a higher carbon content and greater density than brown coal. It is a dense rock of black, sometimes gray-black color with a shiny, semi-matte or matte surface. Contains 75-97% or more carbon; 1.5-5.7% hydrogen; 1.5-15% oxygen; 0.5-4% sulfur; up to 1.5% nitrogen; 45-2% volatiles; the amount of moisture ranges from 4 to 14%; ash - usually from 2-4% to 45%. The higher calorific value, calculated on the wet ash-free mass of hard coal, is not less than 23.8 MJ / kg (5700 kcal / kg).

Coal is the remains of plants that died many millions of years ago, the decay of which was interrupted as a result of the cessation of air access. Therefore, they could not release the carbon taken from it into the atmosphere. The access of air stopped especially sharply where swamps and swampy forests descended as a result of tectonic movements and changes in climatic conditions and were covered from above with other substances. At the same time, plant remains were transformed under the influence of bacteria and fungi (carbonized) into peat and further into brown coal, coal, anthracite and graphite.

According to the composition of the main component - organic matter, coals are divided into three genetic groups: humolites, sapropelites, saprohumolites. Humolites predominate, the source material of which was the remains of higher terrestrial plants. Their deposition occurred mainly in swamps that occupied the low-lying coast of the seas, bays, lagoons, and freshwater basins. As a result of biochemical decomposition, the accumulated plant material was processed into peat, while water content and the chemical composition of the aquatic environment had a significant effect. The carbon content of hard coal ranges from 75 to 90 percent. The exact composition is determined by the location and conditions of coal conversion. Mineral impurities are either in a finely dispersed state in the organic mass, or in the form of the thinnest layers and lenses, as well as crystals and concretions. The source of mineral impurities in fossil coals can be inorganic parts of plants - coal-formers, mineral neoplasms falling out of solutions of water circulating in peat bogs, etc.
As a result of prolonged exposure to elevated temperatures and pressure, brown coals are converted into bituminous coals, and the latter into anthracites. Irreversible gradual change in the chemical composition, physical and technological properties of organic matter at the stage of transformation from brown coal to anthracite is called coal metamorphism.


Structural and molecular rearrangement of organic matter during metamorphism is accompanied by a consistent increase in the relative carbon content in coal, a decrease in oxygen content, and the release of volatile substances; the hydrogen content, combustion heat, hardness, density, brittleness, optics, electricality, and other physical properties change. In the middle stages of metamorphism, coal acquires sintering properties - the ability of gelified and lipoid components of organic matter to pass, when heated under certain conditions, into a plastic state and form a porous monolith - coke. In zones of aeration and active action of groundwater near the Earth's surface, coals undergo oxidation.

In its effect on the chemical composition and physical properties, oxidation has the opposite direction compared to metamorphism:
coal loses its strength properties and sintering properties;
the relative content of oxygen in it increases, the amount of carbon decreases, the humidity and ash content increase, and the calorific value sharply decreases.
The depth of oxidation of fossil coals, depending on the modern and ancient relief, the position of the groundwater table, the nature of climatic conditions, material composition and metamorphism, ranges from 0 to 100 meters vertically.

The specific gravity of coal is 1.2 - 1.5 g / cm3, the calorific value is 35,000 kJ / kg. Coal is considered suitable for technological use if after combustion the ash is 30% or less. Primitive mining of fossil coals has been known since ancient times (, Greece). Coal began to play a significant role as a fuel in Britain in the 17th century. The formation of the coal industry is associated with the use of coal as coke in the smelting of iron. Since the 19th century, a major purchaser of coal has been transport. The main directions of the industrial use of coal: the production of electricity, metallurgical coke, combustion for energy purposes, obtaining various (up to 300 items) products during chemical processing. The consumption of coal is increasing for the production of high-carbon carbon-graphite structural materials, mountain wax, plastics, synthetic, liquid and gaseous high-calorie fuels, aromatic products by hydrogenation, and highly nitrous acids for fertilizers. Coke obtained from coal is needed in large quantities for metallurgical industry.

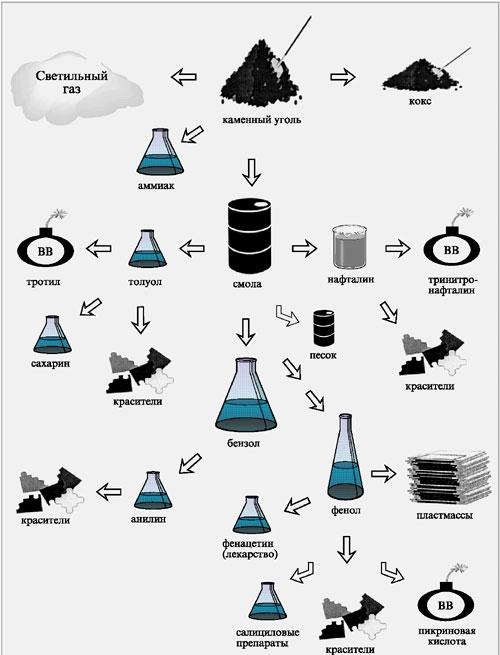
Coke is produced at coke plants. Coal is subjected to dry distillation (coking) by heating in special coke ovens without air access to a temperature of C. In this case, coke is obtained - a porous solid substance. In addition to coke, during the dry distillation of coal, volatile products are also formed, when they are cooled to 25-75 ° C, coal tar, ammonia water and gaseous products are formed. Coal tar undergoes fractional distillation, resulting in several fractions:
light oil (boiling point up to 170 C) it contains aromatic hydrocarbons (benzene, toluene, acids and other substances);
medium oil (boiling point 170-230 C). These are phenols, naphthalene;
heavy oil (boiling point 230-270 C). This is naphthalene and its homologues
anthracene oil - anthracene, fenathrene, etc.
The composition of gaseous products (coke oven gas) includes benzene, toluene, xyols, phenol, ammonia and other substances. Crude benzene is extracted from coke oven gas after purification from ammonia, hydrogen sulfide and cyanide compounds, from which individual hydrocarbons and a number of other valuable substances are isolated.
Amorphous carbon in the form of coal, as well as many carbon compounds, play an important role in modern life as sources of various types of energy. When coal is burned, heat is released that is used for heating, cooking, and for many industrial processes. Most of the heat received is converted into other forms of energy and spent on mechanical work.
Coal is a solid fuel, a mineral of plant origin. It is a dense rock of black, sometimes dark gray color with a shiny matte surface. Contains 75-97% carbon, 1.5-5.7% hydrogen, 1.5-15% oxygen, 0.5-4% sulfur, up to 1.5% nitrogen, 2-45% volatile substances, the amount of moisture ranges from 4 to 14%. The higher calorific value calculated for the wet ashless mass of hard coal is not less than 238 MJ/kg.

Coal is formed from the decomposition products of organic matter of higher plants that have undergone changes under the pressure of various rocks of the earth's crust and under the influence of temperature. With an increase in the degree of metamorphism in the combustible mass, coal increases the carbon content and at the same time reduces the amount of oxygen, hydrogen, and volatile substances. The calorific value of coal also changes.
Characteristic physical properties of coal:
density (g / cm3) - 1.28-1.53;
mechanical strength (kg / cm2) - 40-300;
specific heat capacity C (Kcal / g deg) - 026-032;
refractive index of light - 1.82-2.04.
The largest coal deposits in the world in terms of production volume are the Tunguska, Kuznetsk, Pechora basins - in the Russian Federation; Karaganda - in Kazakhstan; Appalachian and Pennsylvania basins - in the USA; Ruhr - in the Republic of Germany; Big Yellow River - in China; South Wales - in England; Valenciennes - in France, etc.
The use of coal is varied. It is used as household, energy fuel, for metallurgical and chemical industry, as well as for extracting rare and trace elements from it. Coal, coking, heavy industries carry out the processing of coal by coking. Coking is an industrial method of coal processing by heating up to 950-1050 C without air access. The main coke-chemical products are: coke oven gas, crude benzene, coal tar, ammonia.

Hydrocarbons are recovered from coke oven gas by washing in scrubbers with liquid absorption oils. After distillation from oil, distillation from a fraction, purification and re-rectification, pure commercial products are obtained, such as: benzene, toluene, xylenes, etc. From unsaturated compounds contained in crude benzene, coumarone resins are obtained, which are used for the production of varnishes, paints, linoleum and in the rubber industry. A promising raw material is also cyclopentadiene, which is also obtained from coal. Coal - raw material to obtain naphthalene and other individual aromatic hydrocarbons. The most important products of processing are pyridine bases and phenols.
By processing, in total, more than 400 different products can be obtained, the cost of which, in comparison with cost coal itself, increases by 20-25 times, and by-products obtained at coke plants exceed price the coke itself.
Very promising is the combustion (hydrogenation) of coal with the formation of liquid fuel. For the production of 1 ton of black gold, 2-3 tons of coal are consumed. Artificial graphite is obtained from coal. They are used as inorganic raw materials. When processing coal, vanadium, germanium, sulfur, gallium, molybdenum, and lead are extracted from it on an industrial scale. Ash from coal combustion, mining and processing wastes are used in the production of building materials, ceramics, refractory raw materials, alumina, and abrasives. For the purpose of optimal use of coal, it is enriched (removal of mineral impurities).

Coal contains up to 97% of carbon; it can be said that it underlies all hydrocarbons, i.e. they are based on carbon atoms. Often one encounters amorphous carbon in the form of coal. By structure, amorphous carbon is the same graphite, but in a state of the finest grinding. The practical application of amorphous forms of carbon is varied. Coke and coal - as a reducing agent in metallurgy during iron smelting.
Coal classification
Coal is formed from the decomposition products of the organic remains of higher plants that have undergone changes (metamorphism) under the pressure of the surrounding rocks of the earth's crust and relatively high temperatures. With an increase in the degree of metamorphism in the combustible mass of coal, the carbon content consistently increases and at the same time the amount of oxygen, hydrogen, and volatile substances decreases; the heat of combustion, the ability to sinter, and other properties also change. On the change in these qualities, determined by the results of the thermal decomposition of coal (the yield of volatile substances, the characteristic of the non-volatile residue), the industrial classification adopted in the USSR is built.
Coal grades:
long-flame (D),
gas (G),
gas fatty (GZh),
fatty (F),
coke fatty (QOL),
coke (K),
lean sintering (OS),
skinny (T),
weakly caking (SS),
semi-anthracites (PA)
anthracites (A).
Sometimes anthracites stand out in a separate group. For coking, mainly coal grades G, Zh, K and OS, and partially D and T are used. .5-5.0% for grades T-A; decrease in the content (in the combustible mass) of oxygen from 15% to 1.5%; hydrogen - from 5.7% to 1.5%; content sulfur, nitrogen and ash does not depend on belonging to a particular brand. The calorific value of the combustible mass coal increases sequentially from 32.4 MJ/kg (7750 kcal/kg) for grade D to 36.2–36.6 MJ/kg (8650–8750 kcal/kg) for grade K and decreases to 35 .4—33.5 MJ/kg (8450—8000 kcal/kg) for grades PA and A.


According to the size of the pieces obtained during mining, hard coal is classified into:
slab (P) - more than 100 mm,
large (K) - 50-100 mm,
walnut (O) - 26-50 mm,
small (M) - 13-25 mm,
seed (C) - 6-13 mm,
shtyb (W) - less than 6 mm,
ordinary (P) - not limited in size.
Belonging to the brand and the size of pieces of coal are indicated by letter combinations - DK, etc.
Approximately on the same principles as in the USSR, coal classifications are built in a number of Western European countries. IN USA the most common classification is coal, based on the yield of volatile substances and the heat of combustion, according to which they are divided into sub-bituminous with a high yield of volatile substances (corresponds to Soviet grades D and G), bituminous with an average yield of volatile substances (corresponds to grades PZh and K), bituminous with a low yield of volatile substances (OS and T) and anthracite coals, divided into semianthracites (partially T and A), anthracites proper and metaanthracites (A). In addition, there is an international classification of coal, based on the content of volatile substances, caking, coking and displaying the technological properties of coals.
Formation of hard coal
The formation of coal is characteristic of all geological systems, starting from the Silurian and Devonian; coal is very widely distributed in the deposits of the Carboniferous, Permian and Jurassic systems. Coal occurs in the form of seams of various thicknesses (from fractions of m to several tens or more m). The depth of occurrence of coal is different - from the exit to the surface to 2000-2500 m and deeper. With the modern level of mining technology, the extraction of hard coal can be carried out in an open way to a depth of 350 m.
For the formation of coal, abundant accumulation of plant mass is necessary. In ancient peat bogs, starting from the Devonian period, organic matter accumulated, from which, without access to oxygen, fossil coals were formed. Most commercial fossil coal deposits date from this period, although younger deposits also exist. The age of the most ancient coals is estimated at about 350 million years.

Coal is formed when rotting plant material accumulates faster than it can be bacterially decomposed. The ideal environment for this is created in swamps, where stagnant water, depleted of oxygen, prevents the vital activity of bacteria and thereby protects the plant mass from complete destruction. At a certain stage process the acids released during it prevent the further activity of bacteria. This is how peat arises - the original product to form coal. If then it is buried under other deposits, then the peat experiences compression and, losing water and gases, is converted into coal.
Under the pressure of a thickness of sediments with a thickness of 1 kilometer, a layer of brown coal 4 meters thick is obtained from a 20-meter layer of peat. If the depth of burial of plant material reaches 3 kilometers, then the same layer of peat will turn into a layer of coal 2 meters thick. At a greater depth, about 6 kilometers, and at a higher temperature, a 20-meter layer of peat becomes a layer of anthracite 1.5 meters thick.
The method of coal mining depends on the depth of its occurrence. The development is carried out in an open way, if the depth of the coal seam does not exceed 100 meters. There are also frequent cases when, with an ever greater deepening of a coal pit, it is further advantageous to develop a coal deposit by an underground method. Mines are used to extract coal from great depths. The deepest mines in the territory Russia coal is mined from a level of just over 1200 meters.
Along with coal, coal-bearing deposits contain many types of georesources that have consumer significance. These include host rocks such as raw material for the construction industry, groundwater, coal-bed methane, rare and trace elements, including valuable metals and their compounds. For example, some coals are enriched with germanium.
Coal reserves
The general geological reserves of hard coal in the USSR are about 4,700 billion tons (according to estimates in 1968), including grades (in billion tons): D - 1,719; D-G - 331; G - 475; GZh - 69.4; W - 156; QOL - 21.5; K - 105; OS - 88.2; SS - 634; T - 205; T-A - 540; PA, A - 139.
The largest reserves of hard coal in the USSR are in the Tunguska basin. The largest coal basins being developed in the USSR are Donetsk, Kuznetsk, Pechora, Karaganda; V USA- Appalachian and Pennsylvanian, in Poland - Upper Silesian and its continuation in Czechoslovakia - Ostrava-Karvinsky, in Germany— Ruhr, in China— Big Juanhabass, in England— South Wales, in France- Valenciennes and in Belgium - Brabant. The use of coal is varied.

It is used as a household, energy fuel, raw material for the metallurgical and chemical industries, as well as for the extraction of rare and trace elements from it.

For two decades in a row, coal has been in the shadow of the oil boom. Mountains of unmarketable coal grew into the sky. Numerous mines were closed, hundreds of thousands of miners lost theirs. The Appalachian region of the United States, once a flourishing coalfield, has become one of the darkest disaster areas. A disorderly, monopolist-driven transition to cheap, imported - mostly from the Middle East - oil doomed coal to the role of "Cinderella", devoid of a future. However, this did not happen in some countries, including in the former USSR, which took into account the advantages of an energy structure based on national resources.

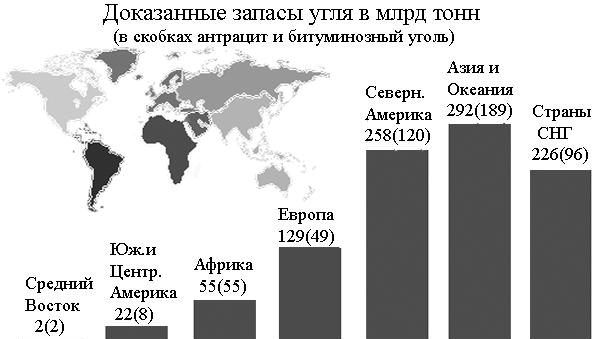
Coal reserves are dispersed throughout the world. Most industrial countries they are not spared. The land is surrounded by two rich coal zones. One stretches through the countries of the former USSR, through China, North America to Central Europe. The other, narrower and less rich, runs from Southern Brazil through South Africa to Eastern Australia.


The most significant deposits coal are located in the countries of the former USSR, the USA and China. Hard coal dominates western Europe. The main coal basins in Eurasia: South Wales, Valenciennes Liege, Saar-Lotharga, Ruhr, Asturias, Kizelovsky, Donetsk, Taimyr, Tunguska, South Yakutia, Funshunsky; in Africa: Jerada, Abadla, Enugu, Huanki, Witbank; in Australia: Great Syncline, New South Wales; in North America: Green River, Junnta, San Juan River, Western, Illinois, Appalachian, Sabinas, Texas, Pennsylvania; in the flaming continent: Carare, Junin, Santa Catarina, Concepción. In Ukraine, the Lvov-Volyn basin and the Donbass rich in deposits should be noted.
Sources
bse.sci-lib.com/ Great Soviet Encyclopedia
en.wikipedia.org Wikipedia - the free encyclopedia
www.bankreferatov.ru abstracts
dic.academic.ru Dictionaries and encyclopedias at Academician
geography.kz Geography
www.bibliotekar.ru Librarian
poddoni.com/ PalletEck
Encyclopedia of the investor. 2013 .
Synonyms:See what "Coal" is in other dictionaries:
Coal- Coal Coal was the first fossil fuel used by man. He enabled the industrial revolution, which in turn helped develop the coal industry by providing it with more modern technology. In 1960 ... ... Wikipedia
